Molecular Neurooncology
Preclinical and clinical trials have demonstrated that oncolytic virotherapy (OVT) is a promising therapy approach to treat solid tumors, and also glioblastomas (GBM). Oncolytic viruses (OVs) can replicate in and subsequently kill tumor cells, but leave non-neoplastic cells un-affected. Additionally, OVs can be armed with therapeutic genes triggering either the patient´s anti-tumor immune response, modulating the GBM microenvironment, or coding for prodrug suicide genes. However, the clinical efficacy of GBM oncovirotherapy has not yet achieved the promising preclinical results. To address this mismatch, one should keep in mind the complex interaction between cancer cells, OV infection and replication, the adjacent tumor microenvironment (TME), chemotherapy as well the patient´s immune system, indicating that not only OVs play a role in an efficient (onco)lysis of GBM cells. OVs are crucial inducers of anti-tumor immune responses and might tilt the suppressive effects of immune evasion mechanisms induced by GBM cells by several mechanisms. Overall, OVs are able to drive anti-GBM immune responses and can initiate anti-GBM immunity.
In a close and long lasting collaboration with the virologist Prof. PS Holm (Universities of Munich and Innsbruck) we analyzed the therapeutic impact of a YB-1 dependent oncolytic adenovirus (XVir-N-31) in vitro as well as in glioma bearing, mice. Injection of orthotopically growing GBM derived from chemotherapy resistant glioblastoma stem cells with XVir-N-31 prolonged the median survival of mice significantly, whereas the chemotherapeutic drug temozolomide (TMZ) that is routinely used to treat GBM had no impact on survival. Brain and lung injections of XVir-N-31 in syrian hamsters, a species susceptible for adenoviral infection and replication, demonstrated that XVir-N-31 is safe and well tolerated since no signs of toxicity as well as no virus replication and virus offspring was detectable in any tissue.
To further optimize OVT, we examined the effects of tumor irradiation on XVir-N-31 based OVT. Irradiation induces an upregulation of YB-1 expression as well as its nuclear translocation, in consequence enhances virus replication in and lysis of GBM cells. Using a human brain slice culture modell, we demonstrated that irradiation prior to virus treatment enhanced tumor cell lysisand reduced the growth as well as tumor cell infiltration into the healthy tissue. Additionally, this combination therapy prolonged the survival of GBM bearing mice.
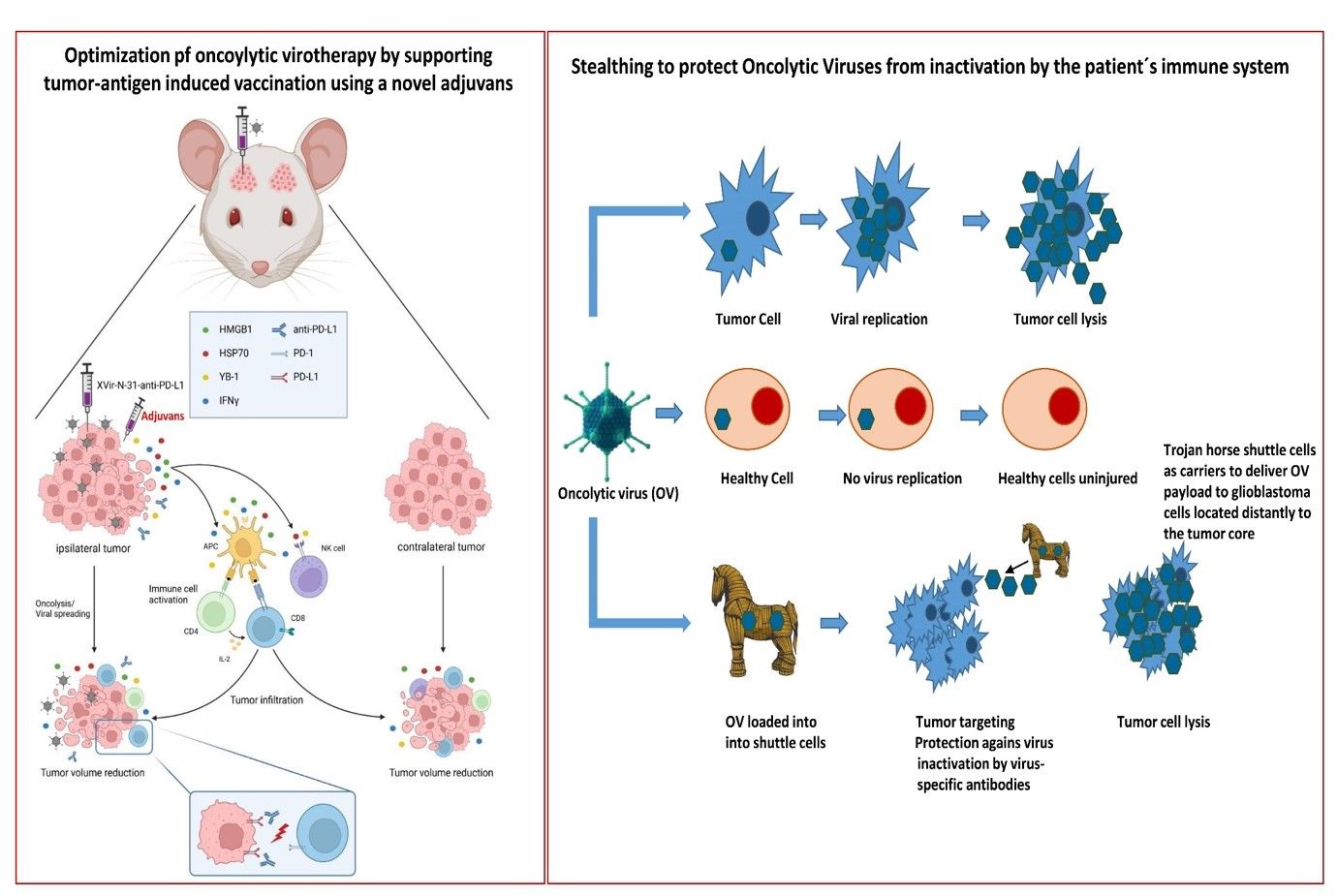
In another project in immunohumanized GBM bearing mice, we combined OVT with immune checkpoint inhibition using XVir-N-31-anti-PD-L1, an derivate of XVIr-N-31 that is armed to express a neutralizing antibody targeting the immune checkpoint protein PD-L1. A single intratumoral injection of XVir-N-31-anti-PD-L1 induced immunogenic cell death (ICD) in the tumor area, led to an elevated number of activated tumor infiltrating lymphocytes (TILs) and reduced the tumor growth significantly. Interestingly, this was no only the case for OV injected GBMs, but also for GBMs that grew in the contralateral hemisphere of the mice, therefore far away from the virus injection side, this mimicking healthy brain infiltrating GBM cells. In this ongoing project we recently started to optimize the OVT-mediated vaccination process that is induced by the release of tumor-(neo)-antigens (TAA) from virus-lysed GBM cells.
Since OAVs have to be delivered intratumorally and therefore might be inactivated by the patient´s immune system, we started, in collaboration with PD Dr. Lusine Danielyan (Clinical Pharmacology, Tübingen), a project that aims to develop an approach that uses intranasal delivery (INA) of shuttle cells loaded with XVir-N-31 as vehicles for the virus transport towards infiltrating glioma cells. Meanwhile we showed that the shuttle cells were efficially attracted by the tumor and reached the location of the tumor and infiltrating tumor cells about 72 h after intranasal application. In further experiments the shuttle cells will be loaded with OAV and the theraputical impact of the INA-based oncolytic virotherapy will be evaluted.