Advanced Cellular Therapies Lab
Our work is dedicated to establish and translate next generation cellular therapeutics to cure childhood cancer. The focus of our work is on addressing limitations of conventional CAR-T technology. To this end, we have developed the adapter CAR-T cell (AdCAR-T) system. By splitting antigen recognition and CAR-T activation, introducing adapter molecules, the system allows precise quantitative (on-/off-switch) as well as qualitative (change and combine target antigens) regulation of CAR-T activity, improving safety and efficacy (antigen evasion). Moreover, AdCAR-T can function as an "OR"gate, allowing simultaneous or sequential multiple targeting, as well as an "AND"gate, capable to identify and differentially lyse target cells based on complex antigen expression profiles. Besides safety concerns and antigen loss, CAR-T activity against solid tumors is significantly hampered by immune evasive mechanisms within the tumor microenvironment (TME). To improve CAR-T function by evidence-based engineering, a deep understanding of the cellular composition of as well as specific immune evasive signatures in the TME is essential. We apply cutting-edge single cell spatial proteomics by ultra-high content imaging to decipher individual immune evasive signatures in primary patient samples as well as complex humanized PDX models.
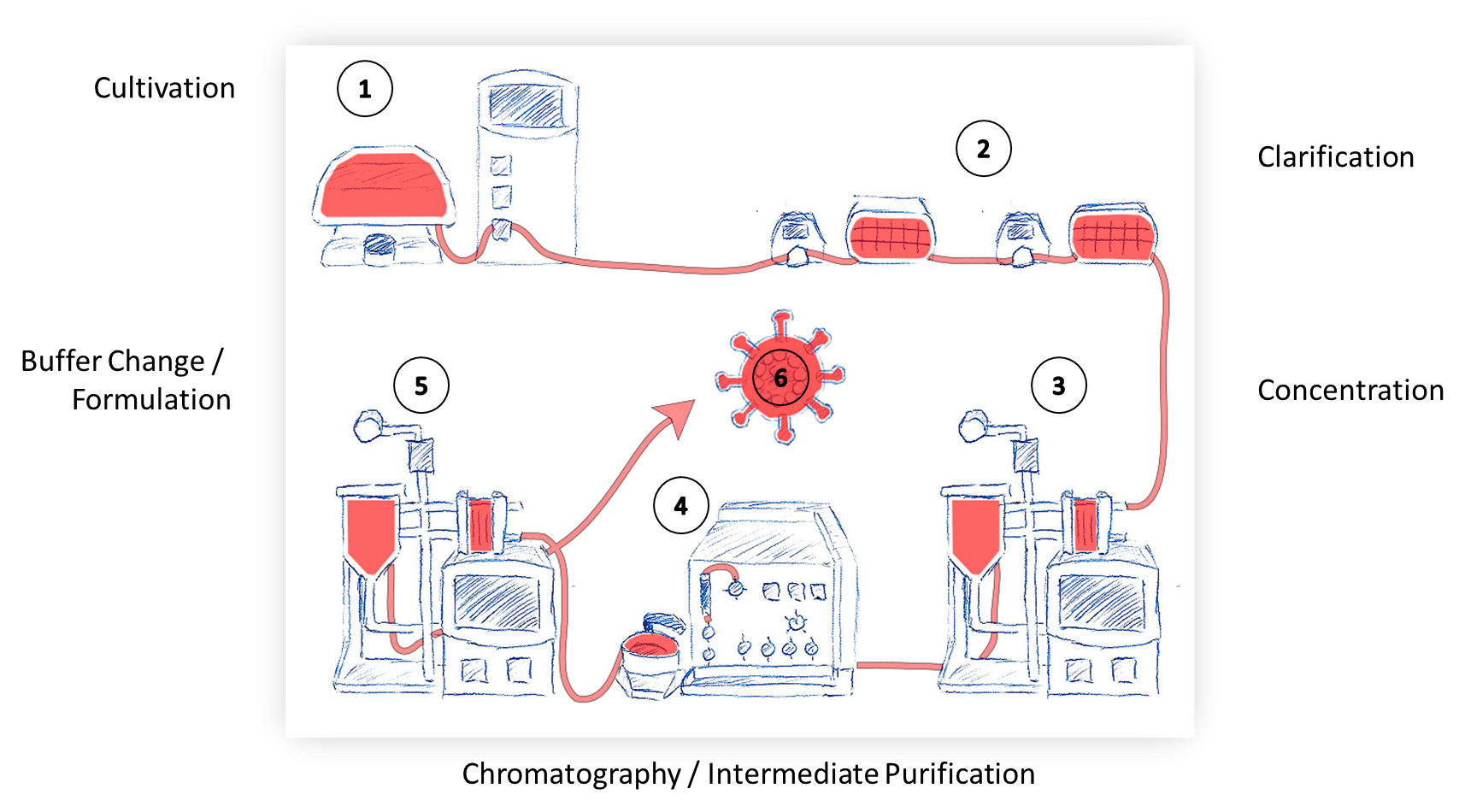
Within the GRT, our group will focus, in close collaboration with other GRT groups, on the establishment, validation and qualification of a GMP-compliant vector production platform. GMP-grade vector (lentiviral or AAV) is essential to genetically modified effector cells, both in adoptive immune cell transfer and gene-replacement strategies. However, the medical need for GMP-grade vector production is currently not met by academia or industry, thereby limiting early phase clinical translation and innovation. Within this consortium, we strive to establish, validate and qualify a closed (all in one line) process for GMP-grade vector (lentiviral or AAV) within our the framework of the GMP-center (GMP-Z) Tübingen. Our goal is to facilitate clinical translation by generating GMP-grade vector (lentiviral or AAV) to GRT investigators.