Arbeitsgruppe Gentherapie für Schwerhörigkeit und Gehörlosigkeit
The Reisinger group aims to refine a gene therapy strategy for DFNB9, the form of deafness caused by mutations in the gene OTOF. Furthermore, we continue to do basic research, aiming to better understand the inner ear function and to identify novel deafness causing genes. This is the basis to develop gene therapy strategies for more forms of deafness, which will hopefully serve to offer a causal and least invasive therapy for more people affected by hearing loss.
Leitung
Head of the group

Prof. Dr. rer. nat. Ellen Reisinger
Phone number: +49 7071-29-88184
E-mail address: ellen.reisinger@uni-tuebingen.de

Contact
frontend.sr-only_#{element.icon}:
Gene Therapy for Hearing Impairment Group
Department of Otolaryngology - Head & Neck Surgery
Elfriede-Aulhorn-Str. 5
72076 Tübingen
frontend.sr-only_#{element.icon}:
André Deutschmann
Dr. rer. nat. Cell Biology
More about the person
frontend.sr-only_#{element.icon}:
Franziska Becker
M. Sc. Biochemistry
More about the person
frontend.sr-only_#{element.icon}:
Xanthoula Smyrnakou-Biedenbänder
M. Sc. Molecular Biotechnology
frontend.sr-only_#{element.icon}:
Ramil Arora
M. Sc.
Areas of Research
- Development of gene therapy for restoration of hearing with adeno-associated viruses
- Basic research to better understand causes and pathogenicity of deafness and progressive hearing impairment
- Regulation of otoferlin by kinases and the impact on synaptic transmission
Expertise
- Transduction of inner ear cells with adeno-associated viruses
- Molecular biology (e.g. cloning, real-time PCR, single cell PCR)
- Design of dual-AAV vectors for large coding sequences
- Cell physiology of inner hair cells (the sensory cells of the inner ear)
- Mouse genetics (Knock-out, knock-In, CRISPR/Cas9 modification of mice)
- Analyses of the synaptic function of inner hair cells by patch clamp
- Immunohistochemistry and confocal fluorescence microscopy

Research Interests
About 0.1% of the population in developed countries are born deaf, which is about 83.000 people in Germany (Deutscher Gehörlosen-Bund e.V.). Including those with hearing impairment and age related hearing loss, about 15 million people in Germany are affected, and more than 400 million worldwide.
Hearing loss can be acquired or innate. Many different forms of hearing impairment are known which are inherited, being caused by mutations in one of about 150 different genes known to date. Presumably several hundred more deafness genes will be identified in near future, e.g. by whole genome sequencing.
People with mild or moderate hearing loss can be helped with hearing aids. In case of a more severe hearing loss, especially if affected people cannot understand speech in everyday life even with hearing aids, cochlear implants are currently the only treatment option. These prostheses can serve to understand speech, such that children can acquire language and visit normal schools. In about 10% of the cases, people with cochlear implants still cannot understand speech. Several research groups of the department study these cases to better understand the causes, aiming to improve the method.
The Reisinger group develops gene therapies for certain forms of hearing impairment. Such a causal therapy would save affected people from an elaborate surgery and from wearing the device. Moreover, people receiving a gene therapy would benefit from a more natural hearing than cochlear implant users, enabling them to better discriminate frequencies, which is a prerequisite to recognize emotions and increases the joy of listening to music. Such a gene therapy requires all cells and cell types of the inner ear to be in place at birth. This is the case for only few forms of deafness, since most products of deafness genes are required during embryonal development of the inner ear.
The Reisinger group aims to refine a gene therapy strategy for DFNB9, the form of deafness caused by mutations in the gene OTOF. This gene encodes the protein otoferlin, which is 1997 amino acids in length. Otoferlin is strongly expressed in inner hair cells, which are the auditory sensory cells in the inner ear. Because DFNB9 is a recessive form of hearing loss, both alleles need to be affected for the hearing loss to show up. With few exceptions, mutations in OTOF lead to prelingual profound hearing loss, but some mutations with moderate or progressive hearing impairment are also known. People with the p.Ile515Thr mutation can perceive sounds even at low sound pressure levels, but their speech comprehension, especially in background noise, is rather poor. Studying a mouse model with this mutation, we found that synaptic transmission from inner hair cells is quickly exhausting, explaining also why people affected by this mutation are not benefiting from hearing aids (Strenzke et al., 2016). More intriguingly, some of the mutations cause a temperature sensitive hearing impairment, such that people have almost normal hearing thresholds at normal body temperature but become profoundly deaf at slightly elevated body temperature, from 38.1°C on (Varga et al., 2006).
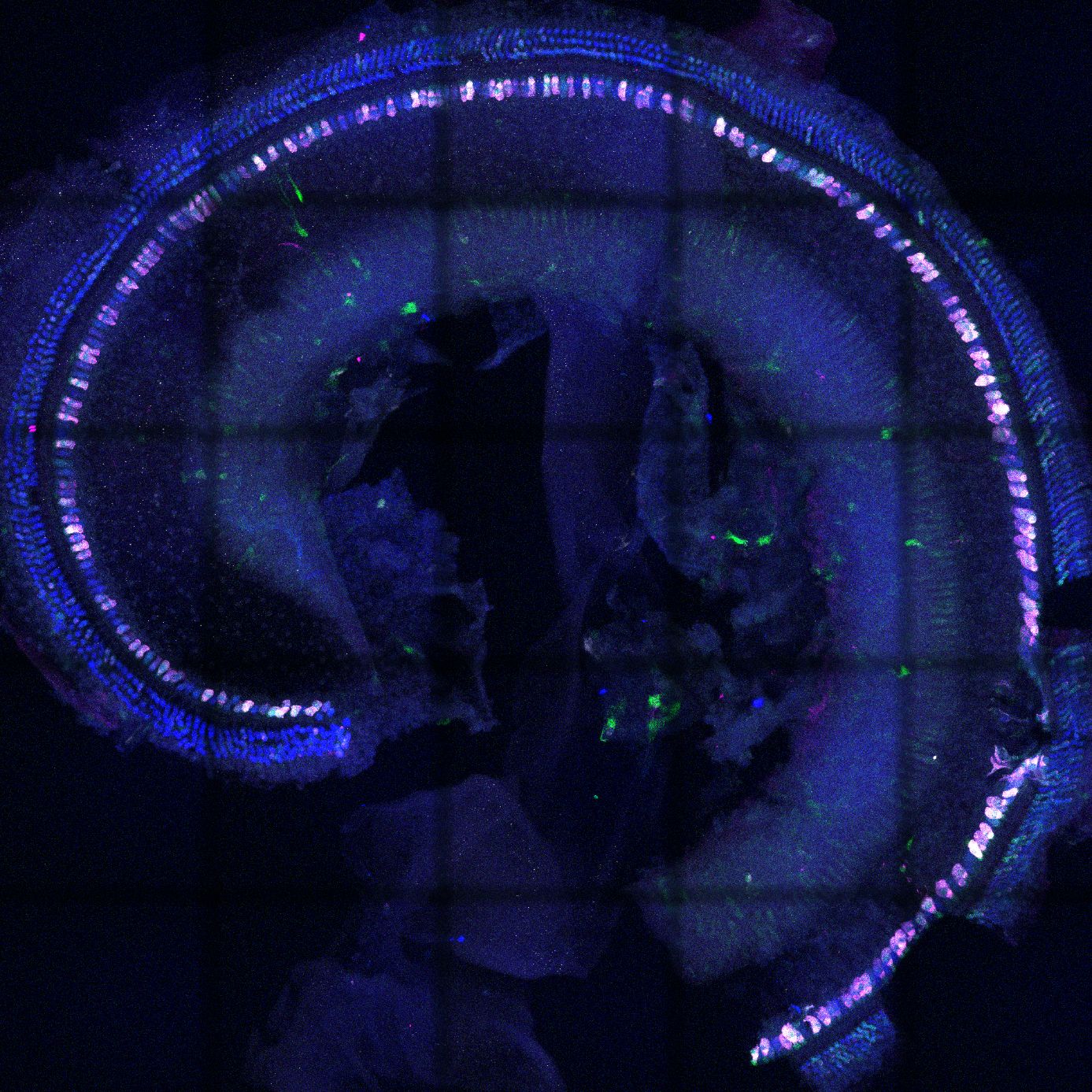
Gene therapy is a causal therapy, by which the missing genetic information of a protein is transmitted to the cells in which this protein is required and normally expressed. Within the cell, the nucleic acids are transported to the nucleus, where they persist and serve to build the correct protein.
In a proof of concept study we showed that two adeno-associated viruses, each transporting one half of the otoferlin coding sequence, restored hearing in a mouse model for DFNB9 (Al-Moyed et al., 2019). The viruses transduced the inner hair cells, where the virus genomes assembled and produced full-length otoferlin mRNA and protein. Hearing was recovered with thresholds of about 50dB, however ABR amplitudes stayed behind wild-type hearing levels. We now aim to improve this dual-AAV strategy to increase both the number of transduced cells and the otoferlin protein levels within the inner hair cells. Our experimental studies contribute to start clinical trials for gene therapy for DFNB9, which are expected to begin in the coming years .
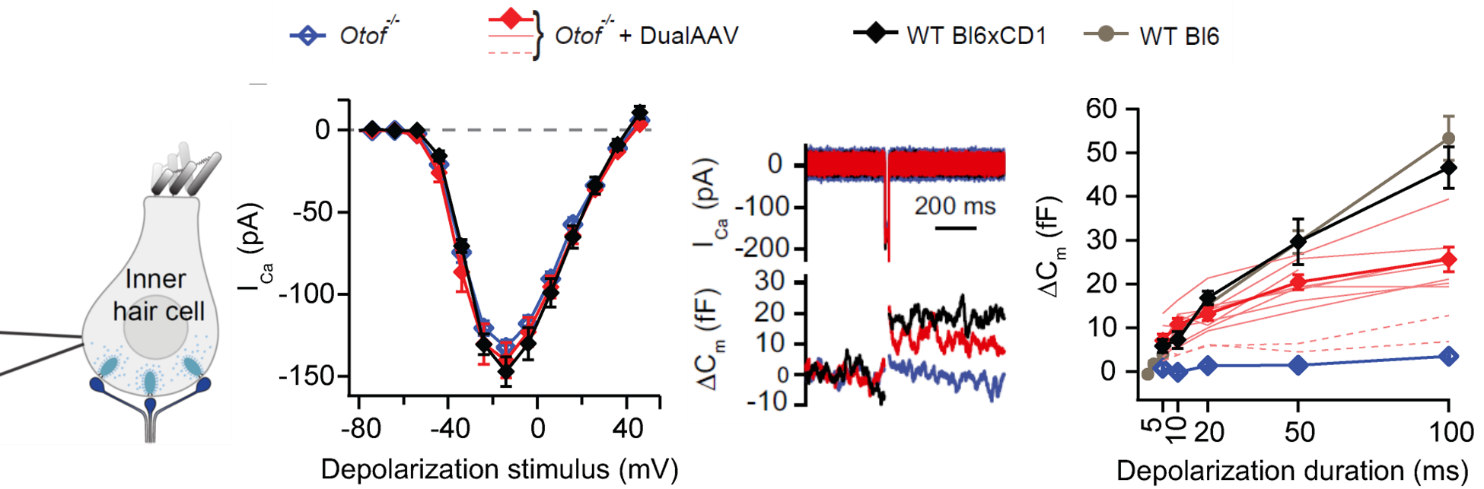
Furthermore, we continue to do basic research, aiming to better understand the inner ear function and to identify novel deafness causing genes. This is the basis to develop gene therapy strategies for more forms of deafness, which will hopefully serve to offer a causal and least invasive therapy for more people affected by hearing loss.
Teaching
Courses with participation of the AG Reisinger
- Graduate Training Center for Neurosciences - Journal Club
- Graduate Training Center for Neurosciences - Sensory Systems II
Certificates and Associations

Focus: Top National Hospital 2025

Stern: Germany's Outstanding Employers in Nursing 24/25

Quality partnership with the PKV

Family as a success factor

Pension provision for the public sector





