Molecular Therapies for Inherited Retinal Dystrophies and Optic Neuropathies
The eye bears a number of important advantages for the development, application and testing of innovative therapies. Consequently, ocular disease such as inherited retinal dystrophies and optic neuropathies are amongst the forerunners in molecular therapy development.
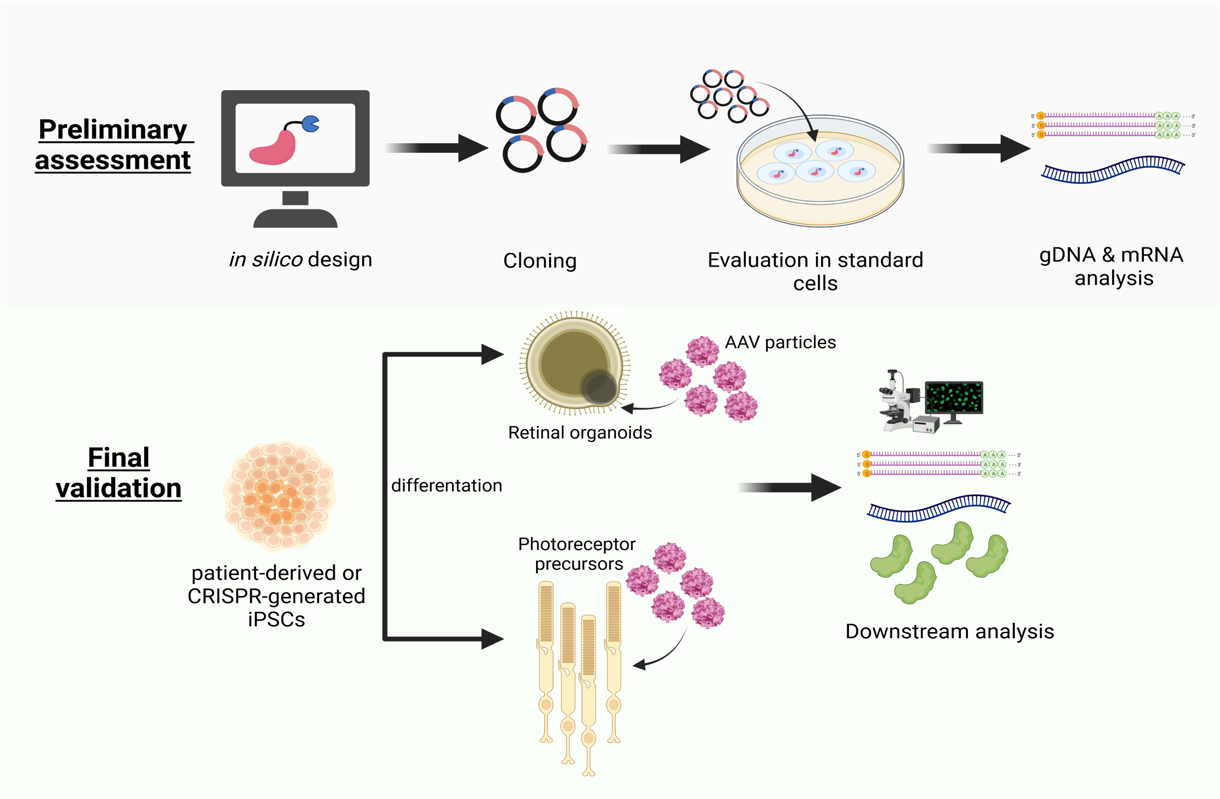
Beyond our contribution to in-house clinical translation programs in supplementation gene therapy for inherited retinal disorders (IRDs), we are exploring and developing innovative genome-editing approaches, which offer safer profile and overcome most of the current hurdles (e.g. use of multiple gRNAs, over activation of P53 pathways, chromosomal aberrations etc.). Specifically, we are applying Enhanced-Deletion RNA-guided endonuclease molecules coupled to single gRNAs to rescue aberrant splicing due to pathogenic (deep-) intronic variants in important IRD genes (ABCA4 and USH2A) as well as to selectively disrupt mutant RHO alleles linked to dominant inherited Retinitis Pigmentosa. Such editing approaches are preclinically validated in patient-derived cellular models. Moreover, we exploit antisense oligonucleotides as therapeutic agents to rescue certain splicing defects linked to IRDs and inherited optic neuropathies.