Our goal is to uncover new molecular mechanisms underlying adipose tissue dysfunction and insulin resistance, which are central contributors to the development of type 2 diabetes.
The research in our group focuses on identifying novel regulators of insulin signaling and lipolysis in adipocytes—two interconnected processes that are often impaired in insulin-resistant states. By leveraging advanced molecular biology approaches, such as the development of genetically encoded biosensors, high-content functional screens, and viral-based gene delivery systems, we aim to dissect the signaling networks and metabolic alterations that accompany insulin resistance. Through this mechanistic understanding, we strive to pinpoint new druggable targets and facilitate the discovery of next-generation insulin sensitizers, ultimately advancing therapeutic options for individuals living with type 2 diabetes.
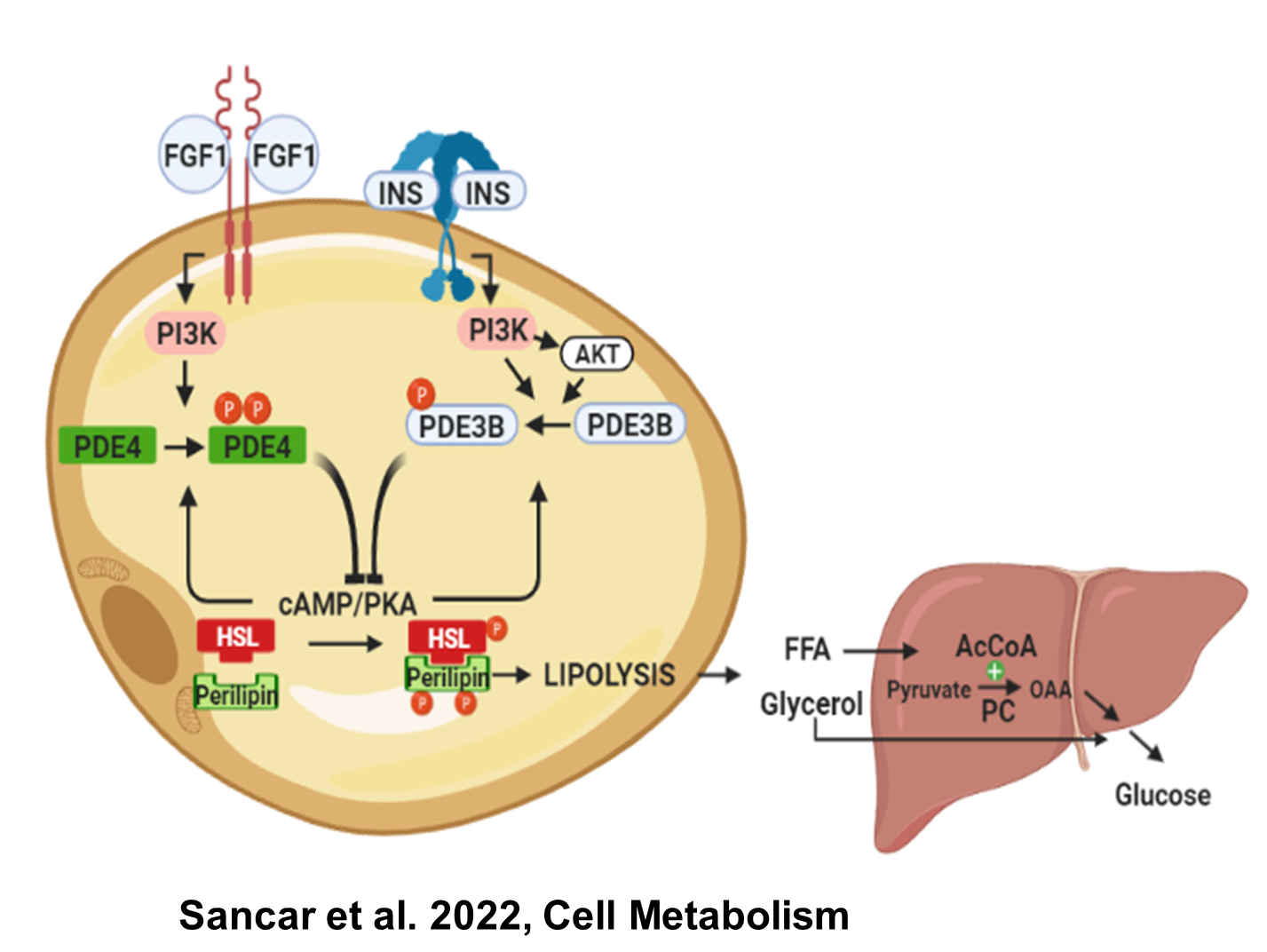
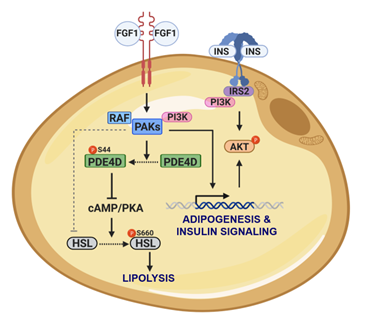
Previously, we identified a distinct FGF1 signaling pathway that mirrors insulin’s ability to suppress adipose lipolysis and hepatic glucose production (HGP). Specifically, FGF1/FGFR1 signaling in adipocytes activates PDE4D, leading to a reduction in cAMP levels and consequently decreased PKA activity. This results in diminished phosphorylation and translocation of hormone-sensitive lipase (HSL), effectively suppressing lipolysis. Now, we focus on further dissecting the signaling mechanisms by which the FGF1/PDE4D axis inhibits lipolysis, and we aim to define the in vivo contribution of adipose PDE4D to metabolic regulation, particularly its role in controlling lipolysis and HGP.
High-fat diet and sedentary lifestyle are key environmental factors that drive unhealthy expansion of adipose tissue and chronic inflammation, leading to adipose tissue dysfunction. Dysfunctional adipose tissue promotes cellular stress, inflammation, and lipotoxicity in other organs such as liver, which in turn exacerbates systemic insulin resistance. This whole-body insulin resistance is a central contributor to the development of prediabetes, type 2 diabetes, and other metabolic complications.