The research laboratory of the Department of Oral and Maxillofacial Surgery focusses the field of bone tissue engineering. We are working with jaw periosteal cells as a “pre-defined” stem cell source and characterize them in detail. Suitable biomaterials will be identified and functionalized with molecules mimicking a natural environment and tested for biocompatibility.
Research
Bone Tissue Engineering
In Germany alone, more than 10.000 people are newly diagnosed with oral cancer every year [1] resulting in tumor and in the most cases jaw bone resection. In order to reconstruct the resulting bone defects, an alternative to autologous bone transplantation is needed. The future-oriented field of tissue engineering (TE) represents a promising alternative therapeutic modality which turns the intrinsic regenerative capacity of the human body into account in order to restore lost tissue function. This procedure includes patient-own stem cells which are optimally predestinated for the required cell type, growing within suitable biomaterials for the generation of 3D TE-constructs.
Research projects
- Generation of induced, pluripotent stem cells from jaw periosteal cells
- Immunogenicity of JPCs / JPC-seeded constructs
- Quality analysis of bone-specific matrix formed by JPCs under serum-containing versus –free culture conditions
- Identification of specific surface marker for osteogenic progenitor cells derived from the jaw periosteum
- Identification and functionalization of an optimal scaffold for JPCs
- Effects of dexamethasone on JPC expression and osteogenic differentiation patterns
- Histological embedding methods and histological/immunohistological stainings of JPC-seeded scaffolds
- Interactions between JPCs and osteoclasts
- Practical work experience
Kooperation Herz-Thorax-Chirurgie - Generierung von induzierten, pluripotenten Stammzellen aus Kieferperiostzellen
DFG-sponsored project / Cooperation with the Department of Thoracic and Cardiovascular Surgery – Generation of induced, pluripotent stem cells from jaw periosteal cells
The reconstruction of large bone resections with BTE products, e.g., for tumor patients, requires extremely high cell numbers. Unfortunately, the production of adequate cell yields is hampered by the occurrence of cell senescence and a decreased osteogenic potential of periosteal stem cells at higher passages. Additionally, primary JPCs exhibit under cultivation with fetal calf serum a high donor variability concerning their differentiation potential.
Although surface markers have been identified, which allow the enrichment of osteogenic progenitor cells from heterogeneous cell populations via fluorescence or magnetic activated cell sorting (FACS, MACS) [8, 16, 19], cell yields are often unsatisfactory because of low sorting efficiencies and high cell mortality.
The use of patient-specific induced pluripotent stem cells (iPSCs) from somatic cells could solve the above-mentioned problems. In the present study, we used for reprogramming of jaw periosteal cells an optimized self-replicating RNA construct containing a green fluorescent protein (GFP) encoding sequence to enable live monitoring of transfected cells. The subsequent differentiation from iPSCs to iMSCs might lead to the generation of a more homogeneous cell population, thus contributing to the standardization of the TE-process.
(PhD student Felix Umrath, Doctoral student Sarah-Lena Frick)
Immunogenicity of JPCs / JPC-seeded constructs
Immunogenicity of JPCs / JPC-seeded constructs
The biocompatibility of in vitro generated tissue engineering constructs has to be tested before their usage in patients. Depending on material characteristics and interactions between stem cells and biomaterial, grafts could initiate an immune reaction.
In order to be able to predict biocompatibility of developed bone constructs, we analyze the interactions between JPCs or JPC-seeded constructs and immune cells (dendritic cells, macrophages) in the co-culture
Following projects are partially sponsored by China Scholarship Council:
- Effects of jaw periosteal cells on the maturation of dendritic cells (PhD student Jingtao Dai)
- Influence of jaw periosteal cells on macrophage differentiation in the two- and three-dimensional (Doctoral student Fang He)
- Establishment of a dynamic co-culture system for the testing of biocompatibility of BTE constructs (Doctoral student Wanjing Cen)
Qualitätsanalyse der während der in vitro JPC-Osteogenese gebildeten Extrazellulärmatrix
Quality analysis of bone-specific matrix formed by JPCs under serum-containing versus –free culture conditions
The clinical application of in vitro developed tissue engineering constructs in humans makes a standardization of the manufacturing process necessary. In terms of biomaterials, the innovative 3D printing technology allows a high level of precision and uniformity of printing quality leading to standardized fabrication of 3D scaffold materials. However, material modification with functional bio-molecules for enhancement and controlling of cell function require a suitable detection/quantification method which is still lacking at present. At the same time, maturation of in vitro generated cell-material constructs has to be recorded step by step by quantitative live-imaging and monitoring for quality control. This should assure the success of the TE procedure and determine the optimal time point for construct implantation.
Jaw periosteal cells are able to form bone-specific matrix after stimulation with specific agents. Cell mineralization can be detected using histologic staining and quantification. However, no statements concerning the quality of the formed matrix can be done. Using the non-destructive Raman technology, fingerprint Raman spectra containing information about biochemical composition of the formed matrix can be generated, based on molecule vibrations. The biochemical characterization of mineralized tissues provides structural and functional information, which might help to classify and discriminate formation of poor quality bone tissue.
DFG sponsoring Differences in the biochemical composition of mineralized species formed by MSCA-1+ JPC-subpopulation under serum-containing and serum-free culture conditions were analyzed. Under serum-free culturing, MSCA-1+ JPCs form a more mature bone-like matrix which probably possesses less elastic properties due to a reduced collagen formation by the cells. [13]
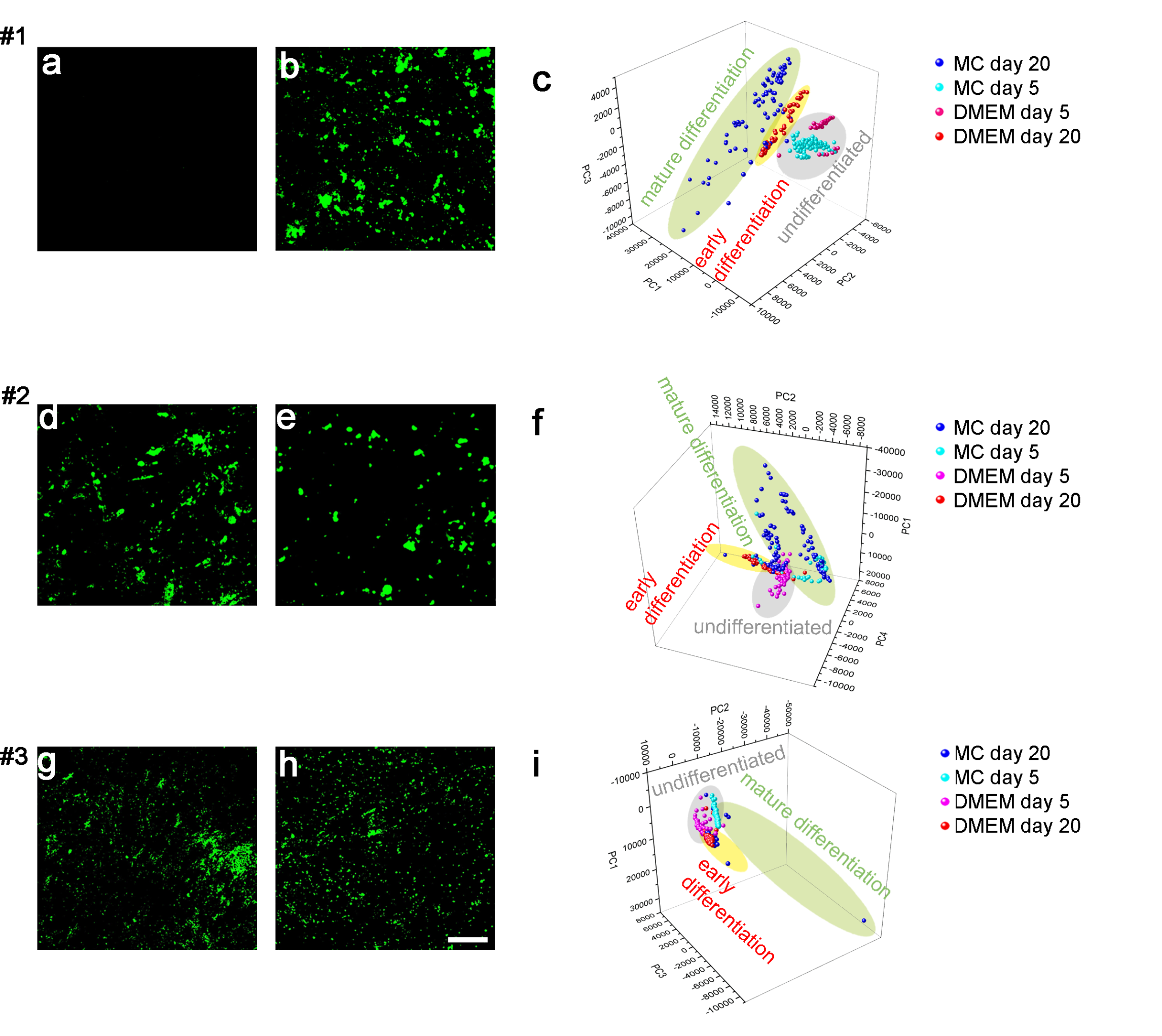
In this study we combined atomic force microscopy with raman spectroscopy in order to characterize both mechanical and biochemical properties of the formed mineralized matrix, at the same time. For future clinical applications it is important to remove animal components from in vitro cell culture. Therefore, we compared mineralized cell monolayers under FCS- and hPL-supplementation and found evidence for a high-quality matrix under hPL-supplementation [15].

Identifizierung von spezifischen Oberflächenmarkern für die osteogenen Vorläuferzellen aus dem Kieferperiost
Identification of specific surface marker for osteogenic progenitor cells derived from the jaw periosteum
BMBF-sponsoring / DFG sponsoring Jaw periosteum is a very thin two-layer membrane. The outer layer contains mainly tissue fibroblasts while the inner layer facing the bone tissue contains mainly osteoprogenitor cells.
Our in vitro cell culture is a mixed population containing cells from both cell layers due to failed separation of them.
We identified mesenchymal stem cell antigen-1 (MSCA-1) as being specific for the subpopulation from jaw periosteum with the highest osteogenic capacity. Therefore, MSCA-1 subpopulation can be easily isolated [16, 19]. Unfortunately, fluorescence and magnetically-activated cell sorting is accompanied by high mortality rates. Interestingly, the in vitro cultivation with a serum-free medium led to a certain selection of the MSCA-1+ subpopulation [6].

Identifizierung und Funktionalisierung eines optimalen Gerüstmaterials für JPCs
Identification and functionalization of an optimal scaffold for JPCs
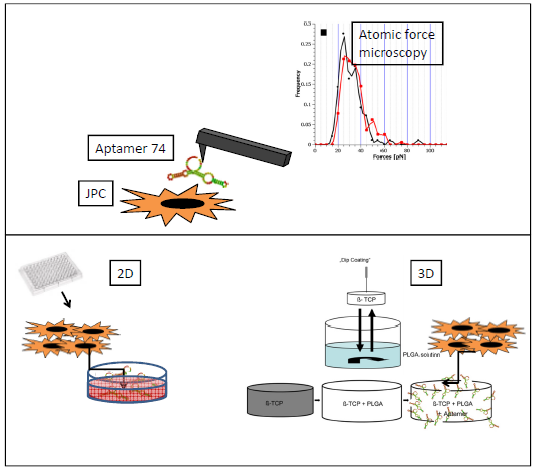
AiF sponsoring Different degradable biomaterials will be tested for suitability for jaw periosteal cells [5].
In order to mimic a natural microenvironment on synthetically manufactured biomaterials, different strategies for biofunctionalization and immobilization will be chosen [9-12].
Effects of dexamethasone on JPC expression and osteogenic differentiation patterns
Effects of dexamethasone on JPC expression and osteogenic differentiation patterns
(Doctoral student Achim Pfeifer)
Histological embedding methods and histological/immunohistological stainings of JPC-seeded scaffolds
Bachelor thesis for Medical Technology (Bachelor student Lukas-Frank Schmitt)
Interactions between JPCs and osteoclasts
Master thesis for Medical Technology (Master student Alena Fischer)
Practical work experience
Cooperation with the University of Sassari/Sardinia (Erasmus student Stefano Carta)
Certificates and Associations

Focus: Top National Hospital 2025

Stern: Germany's Outstanding Employers in Nursing 24/25

Quality partnership with the PKV

Family as a success factor

Pension provision for the public sector






