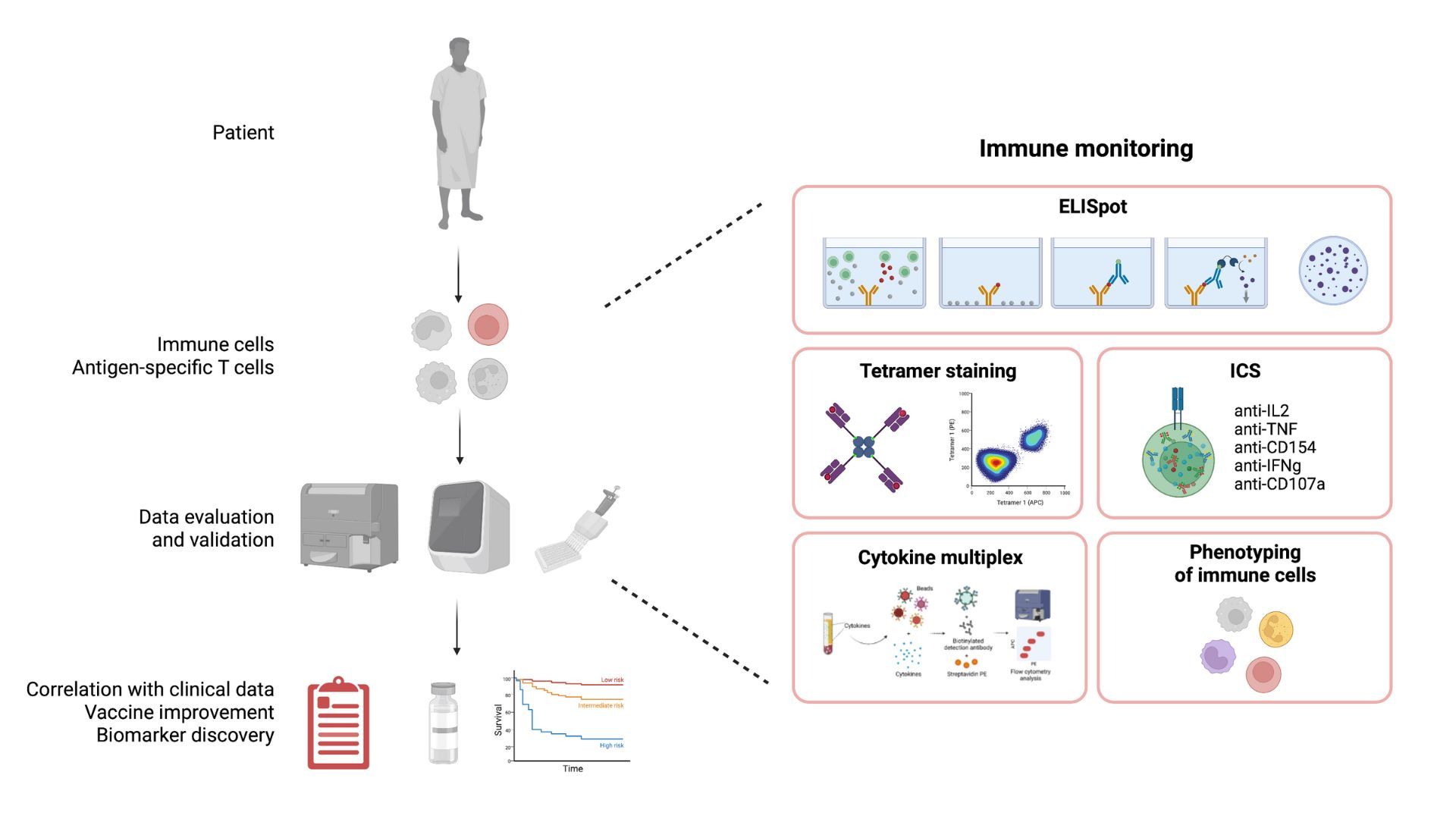
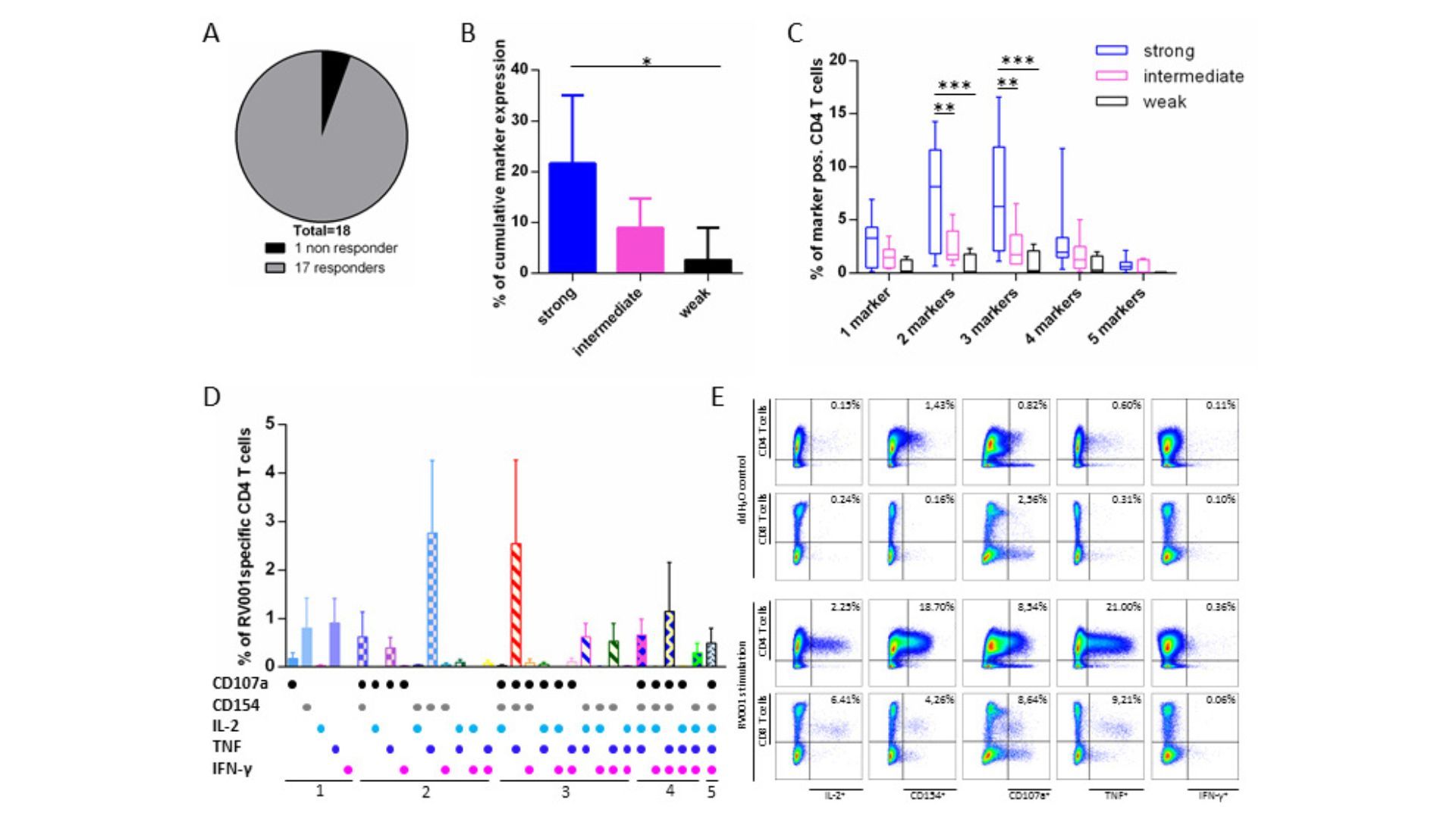
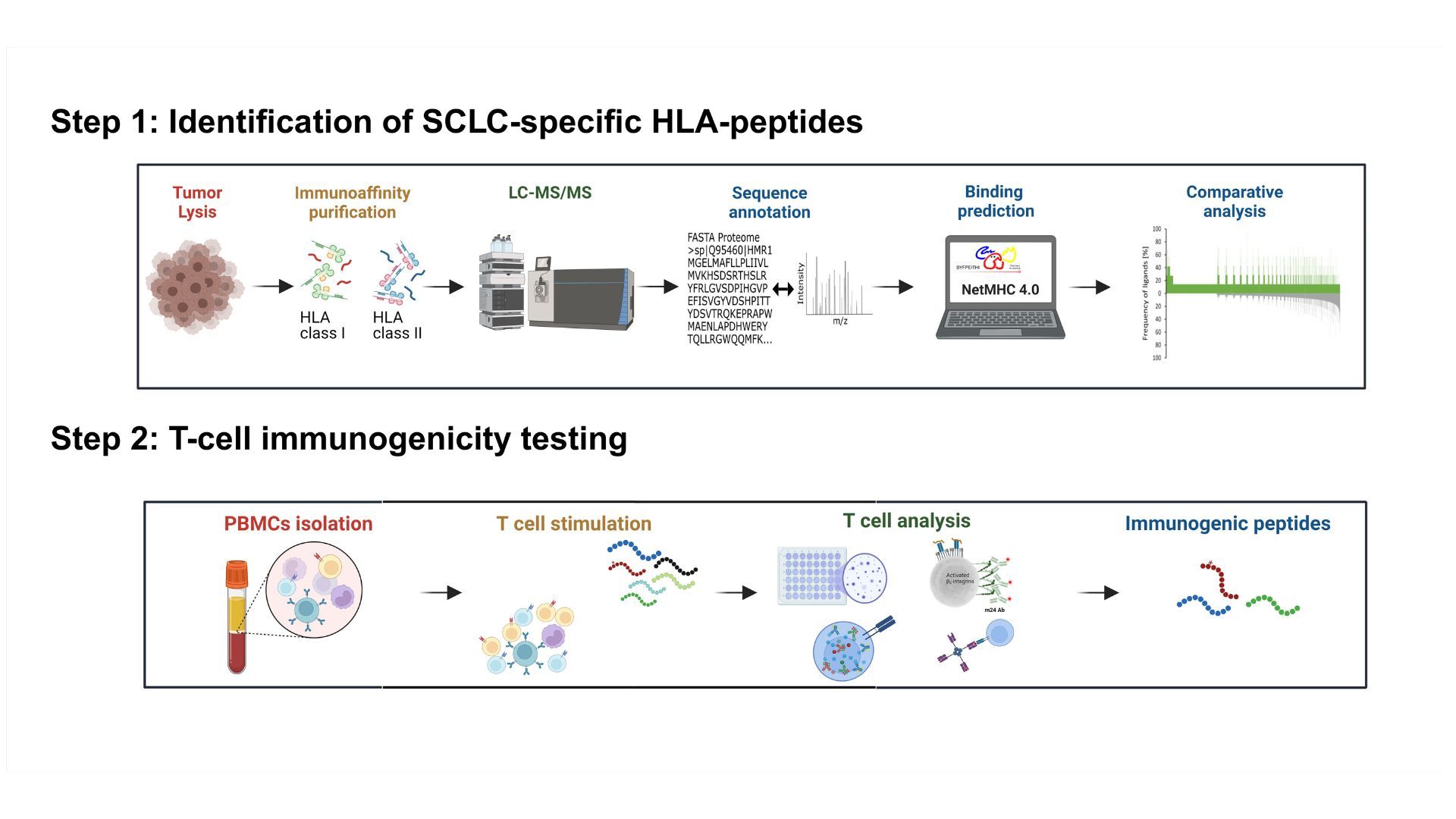
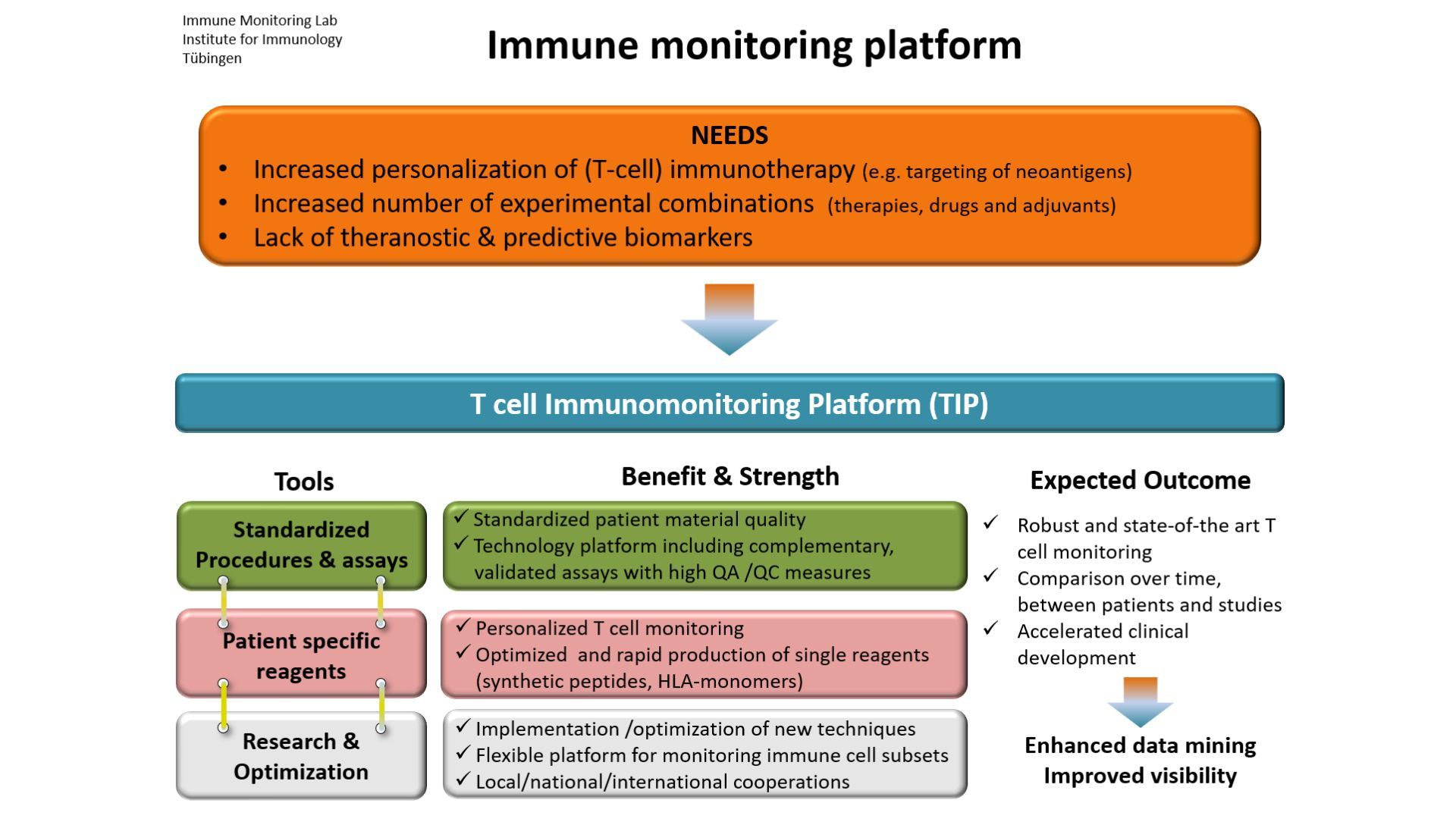
The aim of the immune monitoring team is to contribute to the understanding of immune responses against cancer and to apply this knowledge in immunotherapy. We are focusing on the description of anti tumor T cell responses in cancer patients, either before or during therapy, including immunotherapy. We investigate subsets of T cells at the phenotypic and functional levels, in the blood of cancer patients and also within the tumor tissue. We also assess other immune cell subsets interacting with human tumors and their impact on T cells and clinical course. Finally, we are currently identifying tumor antigens for small cell lung carcinoma.
Key Areas:
- T cells
- Cancer
- Immunotherapy
- Immunomonitoring