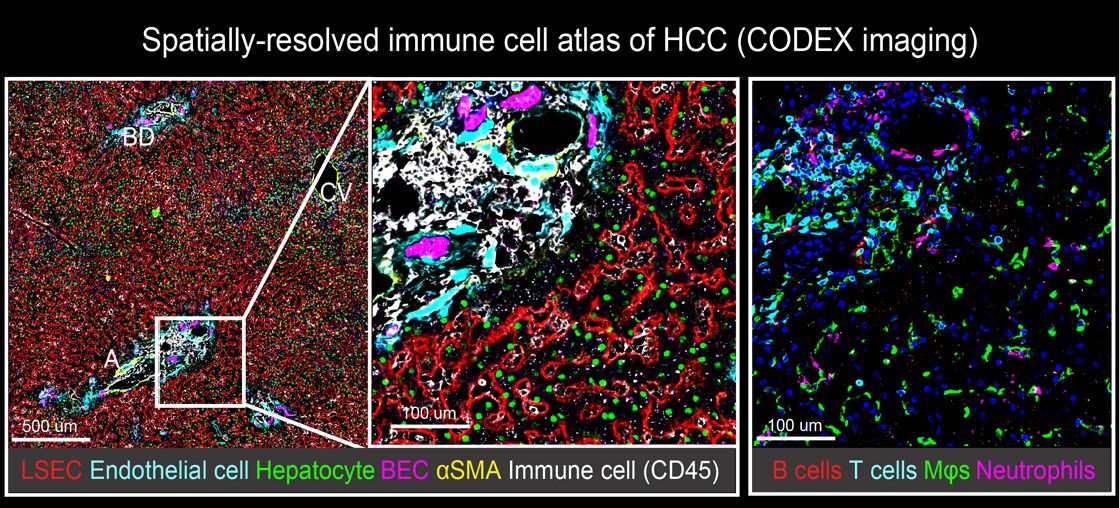
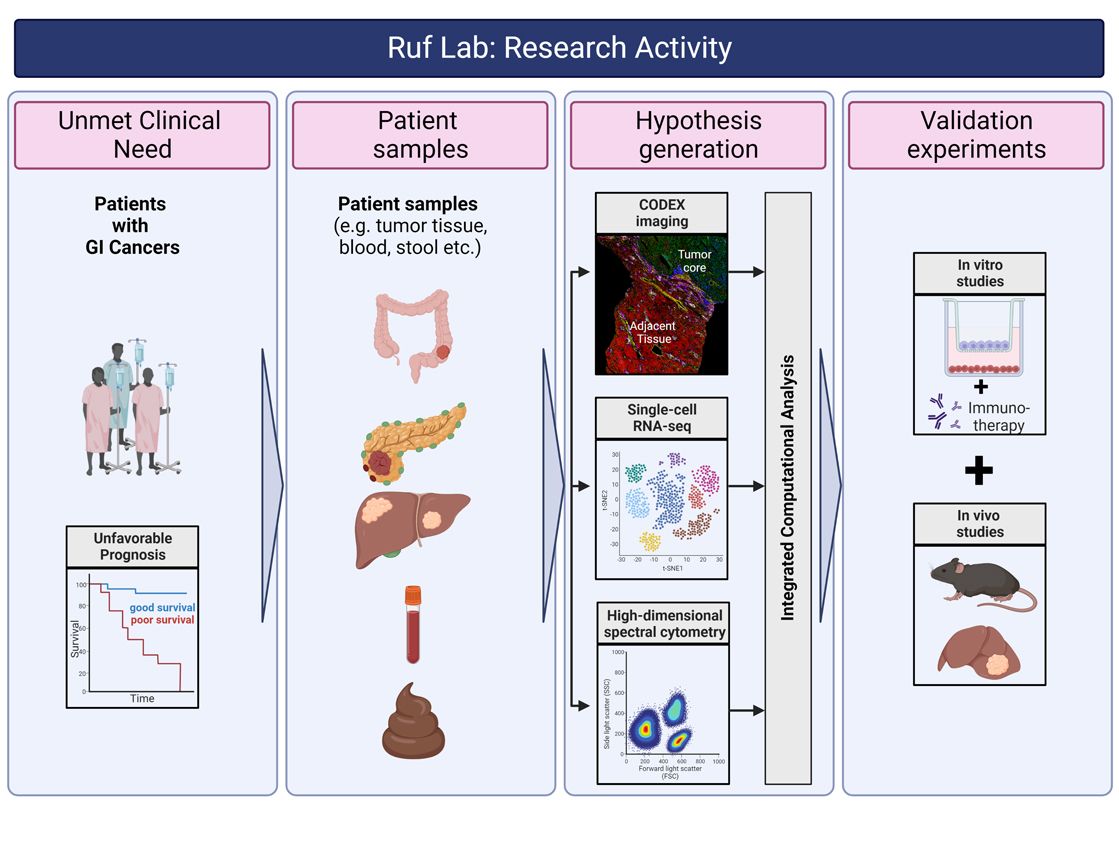
In our lab, we aim to expand our knowledge of the composition, phenotype, and organization of the tumor immune microenvironment (TiME) in gastrointestinal cancers with the goal to ultimately improve patient care. Our research is located at an exciting interface between basic immunology, translational and clinical research. We use clinical data to guide our research focus, we use systems biology approaches to build hypotheses and then test these hypotheses in functional in vivo and in vitro models. We employ complementary experimental strategies to investigate the TiME in patient samples including scRNA-seq, spatial proteomics and high-dimensional spectral cytometry. Using these approaches, we study how reciprocal communications between the innate and adaptive immune system, stomal cells and cancer cells are altered in response to tumor initiation, progression, and response to (immuno)therapy. One focus of our research program is to investigate the role of tissue-resident lymphocytes, in particular mucosal-associated invariant T (MAIT) cells, in the context of cancer. Beyond that and following up on previous studies, we continue to explore how the liver as a site of primary tumorigenesis or metastasis represents a unique tumor microenvironment that requires special consideration.
The overarching goal of our research is to translate our findings to the clinic and to develop prudent immunotherapeutic strategies to disrupt the hostile TiME for the benefit of patients with gastrointestinal cancers.