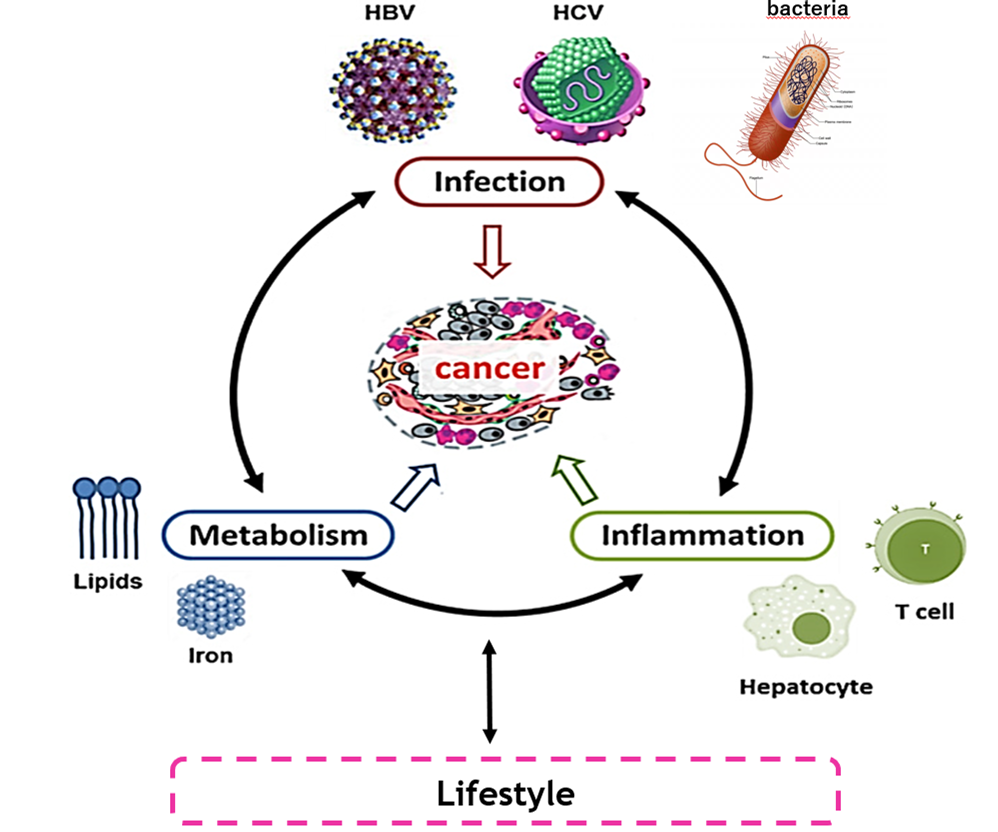
The laboratory of Mathias Heikenwälder aims at understanding the different immune signatures of chronic inflammatory human diseases driving cancer - with the final aim to generate appropriate mouse models used for pre-clinical research and translation into the clinic. A focus of the laboratory is to understand how inflammation (induced by life-style factors or pathogens (e.g. viruses or bacteria)) drives cancer in regenerative organs (such as the liver or the gastrointestinal tract).
A particular research focus of the Mathias Heikenwälder laboratory is the elucidation of the molecular and cellular mechanisms causing fatty liver disease, subsequent inflammation, tissue damage (called non-alcoholic steatohepatitis (NASH)) and resulting liver cancer. Life-style related factors have contributed to the fact that today liver cancer is the 4th most common cause for cancer-related death and the fastest rising cancer in the world.