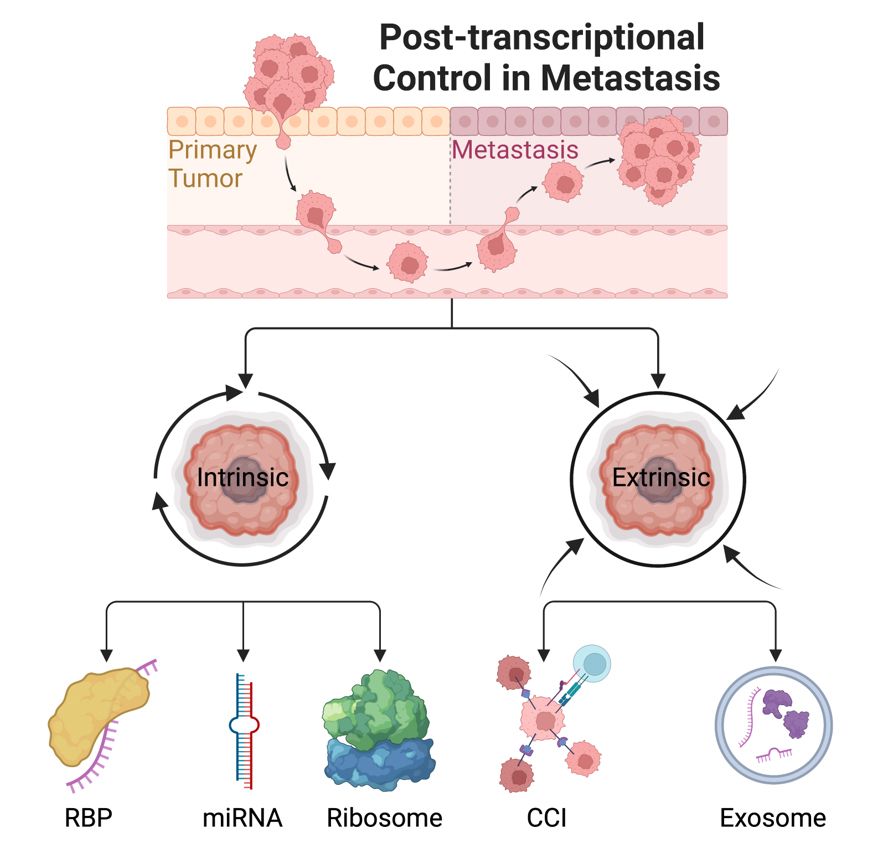
Metastatic disease remains the leading cause of cancer-related deaths worldwide, accounting for over 90% of fatalities. Unfortunately, there is still a significant lack of therapies specifically targeting the process of metastasis. Our research group leverages state-of-the-art high-throughput sequencing technologies to investigate the impact of post-transcriptional gene regulation mediated by microRNAs (miRNAs) and RNA-binding proteins (RBPs) on the development of metastases. We employ cutting-edge techniques in molecular research to elucidate the intricate mechanisms of metastasis in both cellular and murine models. Our primary objective is to identify both cell-intrinsic (autonomous) and cell-extrinsic (nonautonomous) mechanisms involved in post-transcriptional gene regulation in the process of metastasis (See Fig.). By translating our newly acquired knowledge into clinical practice, we aim to facilitate the development of innovative therapies to effectively target the formation of metastatic spread.
Post-transcriptional control in metastasis

Head of the research group
Publications: Pubklikationen
Biosketch
Dr. Missios holds a degree in Human Medicine from the University of Ulm, Germany, where he completed his state examination in November 2012. Under the supervision of Karl Lenhard Rudolph he pursued his MD-Ph.D. studies and graduated with honors (summa cum laude) in May 2015. From 2013 to 2015. Dr. Missios completed his Residency in Internal Medicine at the University Hospital Tuebingen. Next, he joined George Q. Daley’s lab at the Stem Cell Program, Boston Children's Hospital, Harvard Medical School in Boston, MA, USA. His postdoctoral training went from 2015 to 2019. In 2019, he returned to Tuebingen as a Group leader in the field of Posttranscriptional control in advanced malignancy. Concurrently, he served as a clinical fellow in the Department of Internal Medicine I of the University Clinic Tuebingen. He successfully competed his Fellowship in Internal Medicine with a specialization in Gastroenterology and Gastrointestinal Oncology at the University Hospital Tuebingen in February 2023. Apart from his research, he assumes clinical duties as an attending physician at the oncology day clinic.
Selected publications
- Missios, Pavlos, Edroaldo Lummertz da Rocha, Daniel S. Pearson, Julia Philipp, Maria M. Aleman, Mehdi Pirouz, Dorian Farache, u. a. „LIN28B Alters Ribosomal Dynamics to Promote Metastasis in MYCN-Driven Malignancy“. Journal of Clinical Investigation 131, Nr. 22 (15. November 2021): e145142. https://doi.org/10.1172/JCI145142.
- Bitzer, Michael, Stephan Spahn, Sepideh Babaei, Marius Horger, Stephan Singer, Klaus Schulze-Osthoff, Pavlos Missios, u. a. „Targeting Extracellular and Juxtamembrane FGFR2 Mutations in Chemotherapy-Refractory Cholangiocarcinoma“. Npj Precision Oncology 5, Nr. 1 (Dezember 2021): 80. https://doi.org/10.1038/s41698-021-00220-0.
- Missios, Pavlos, Janina Beha, Michael Bitzer, und Nisar P. Malek. „Das molekulare Tumorboard“. Der Chirurg 92, Nr. 11 (November 2021): 1011–15. https://doi.org/10.1007/s00104-021-01487-6.
- Eser, Pınar Özden, Raymond M. Paranal, Jieun Son, Elena Ivanova, Yanan Kuang, Heidi M. Haikala, Ciric To, Jeffrey J Okoro, Kshiti H Dholakia, Jihyun Choi, Yoonji Eum, Atsuko Ogino, Pavlos Missios u. a. „Oncogenic Switch and Single-Agent MET Inhibitor Sensitivity in a Subset of EGFR -Mutant Lung Cancer“. Science Translational Medicine 13, Nr. 609 (September 2021): eabb3738. https://doi.org/10.1126/scitranslmed.abb3738.
- Franses, Joseph W., Julia Philipp, Pavlos Missios, Irun Bhan, Ann Liu, Chittampalli Yashaswini, Eric Tai, u. a. „Pancreatic Circulating Tumor Cell Profiling Identifies LIN28B as a Metastasis Driver and Drug Target“. Nature Communications 11, Nr. 1 (Dezember 2020): 3303. https://doi.org/10.1038/s41467-020-17150-3.
- Tao, Ting, Hui Shi, Luca Mariani, Brian J. Abraham, Adam D. Durbin, Mark W. Zimmerman, John T. Powers, Pavlos Missios, u. a. „LIN28B Regulates Transcription and Potentiates MYCN-Induced Neuroblastoma through Binding to ZNF143 at Target Gene Promotors“. Proceedings of the National Academy of Sciences 117, Nr. 28 (14. Juli 2020): 16516–26. https://doi.org/10.1073/pnas.1922692117.
- Rowe, R. Grant, Edroaldo Lummertz da Rocha, Patricia Sousa, Pavlos Missios, Michael Morse, William Marion, Alena Yermalovich, u. a. „The Developmental Stage of the Hematopoietic Niche Regulates Lineage in MLL-Rearranged Leukemia“. Journal of Experimental Medicine 216, Nr. 3 (4. März 2019): 527–38. https://doi.org/10.1084/jem.20181765.
- Missios, Pavlos, Yuan Zhou, Luis Miguel Guachalla, Guido von Figura, Andre Wegner, Sundaram Reddy Chakkarappan, Tina Binz, u. a. „Glucose Substitution Prolongs Maintenance of Energy Homeostasis and Lifespan of Telomere Dysfunctional Mice“. Nature Communications 5, Nr. 1 (Dezember 2014): 4924. https://doi.org/10.1038/ncomms5924.