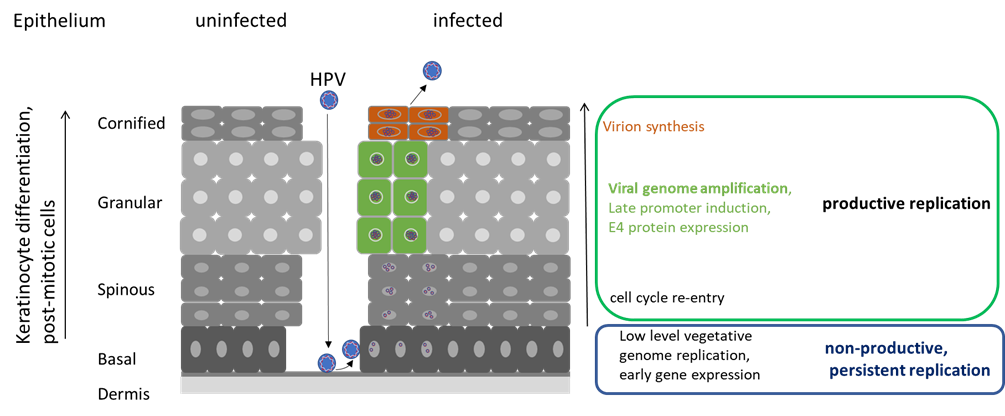
HPV infect and complete their replication cycle in keratinocyte cells that are the main constituents of mucosal and cutaneous epithelium. In basal epithelial cells low levels of HPV genome replication and expression of early viral gene products can be observed. Production of infectious HPV occurs only in the upper layers of the infected epithelium.
- We aim to understand the regulation of the different phases of the HPV replication cycle in order to identify key regulatory events that can be targeted by antivirals.
- We also aim to understand the differences between high risk HPV and beta-HPV
The HPV genome is a double-stranded, covalently closed DNA molecule of 8kbp that is packaged in a chromatinized form. The viral E1 and E2 proteins are the key replication activators and form a complex that recognizes the viral origin. E1 acts as DNA helicase and recruits host cell proteins to initiate the replication of the genome. E2 is not only a loader for the E1 helicase but also modulates viral transcription and acts as segregation factor for the viral genomes upon cell division.
Our studies have uncovered that HPV encode E8^E2, an alternative E2 protein, limits viral replication in undifferentiated cells by repressing viral transcription and E1/E2-dependent DNA replication. Using a panel of different technologies (immunoprecipitation-mass spectrometry, fluorescence microscopy, RNA interference, transcription and replication reporter assays), we have identified the cellular NCoR/SMRT repressor complex as a mediator of E8^E2´s repression activities. Our recent in vivo studies have revealed that E8^E2 prevents the switch to the productive phase in undifferentiated cells which is incompatible with cell division and therefore tumor formation in vivo. This suggests that the E8^E2-NCoR/SMRT complex interaction might be an antiviral target.
- we investigate the molecular repression mechanism by the E8^E2-NCoR/SMRT complex
- we investigate the regulation of E2 and E8^E2 proteins by post-transcriptional mechanisms such as post-translational modifications (phosphorylation, acetylation, etc.) in the non-productive and productive phase of the viral replication cycle
- we investigate the interactome of E2 proteins involved in transcription control, activation of DNA replication, and genome partitioning