Virotherapie Center Tübingen (VCT)
The group of Prof. Ulrich M. Lauer is focusing on preclinical as well as clinical research on oncolytic virotherapy. Oncolytic viruses (OV) specifically replicate in cancer cells and thereby destroy cancer tissues but do not harm normal cells. Many OVs exert their anti-tumor effect not only by directly lysing the cancer cells, but also by stimulating the anti-cancer immune response as a consequence of cell lysis and release tumor specific antigens in a virus-induced inflammatory tumor microenvironment. By these ways, OVs are able to lead to cancer regression also in patients for whom standard therapies have failed. Furthermore, OV-therapy can be combined with standard therapies (e.g. chemo-/immunotherapy or irradiation), making them exciting and highly promising new anticancer agents. Especially genetically modified OVs, which overcome the limitations of 1st generation wild-type viruses, have shown great potential in the treatment of cancer.
Together with scientists from the Max-Planck-Institute (MPI) of Biochemistry in Martinsried (Munich) (Prof. Wolfgang Neubert) the group of Prof. Lauer has generated a suicide gene-armed virotherapeutic vector which greatly enhances the oncolytic efficiency when treating different kinds of tumors (such as non-small cell lung, colon, melanoma, ovarian, renal, prostate, breast cancer and many others). All CMC activities already have been established and validated and an academic / investigator-initiated phase 1 trial is in preparation.
Furthermore, numerous Phase I/II virotherapy trials are carried out at the Tübingen Early Clinical Trials Unit (ECTU; headed by Prof. Lauer), making it one of Germany´s top Clinical Virotherapy Centers.
In anticipation of future even more personalized virotherapy, experimental systems that mimic the response of solid tumors to oncolytic agents have been established based on patient-derived three-dimensional organoid models. Hallmark parameters such as efficiencies of virotherapeutic infections, kinetics of intratumoral viral replication and immune-mediated oncolysis are determined in vitro. This will not only help to test and further engineer new generations of virotherapeutic vectors, but also to predict their efficacies in a highly personalized context (in the sense of a companion diagnostic).
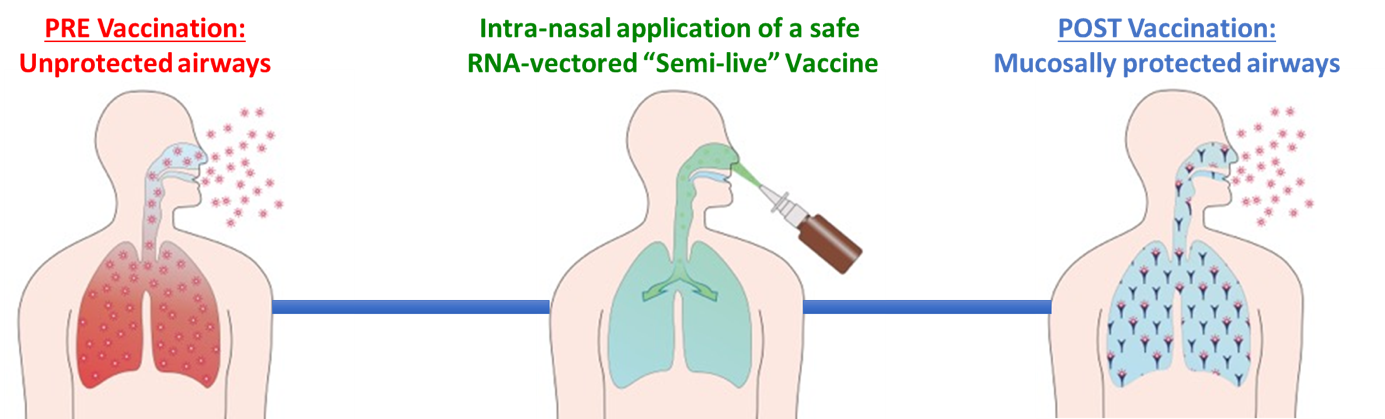
In a further project, the group of Prof. Ulrich M. Lauer is working on viral vector-based vaccines against pandemic threats caused by viruses such as SARS-CoV-2 (COVID-19) or other respiratory pathogens. In collaboration with the MPI-B (Prof. Reinhard Fässler, Dept. Molecular Medicine) novel viral vector-based “Semi-live” vaccines (the “vir4vac” platform) are generated for intranasal use against SARS-CoV-2 (COVID-19) or other respiratory pathogens. For this purpose, a unique respiratory viral vector (Sendai virus-based), which copies the natural way of respiratory infections, is employed to express the Spike (S) protein of SARS-CoV-2. This results in a full protection also of the airways and thus fully breaks any further transmissions of SARS-CoV-2, thereby preventing any uncontrolled infections. According to WHO, such vaccines are classified as 2nd generation vaccines and are supposed to be superior, especially in inducing an effective sterilizing immunity, when compared with any 1st generation types of anti-Covid-19 vaccines which only can be applied i.m. so far.