The research group led by Katrin Böttcher explores how immune cell function is modulated by tissue-specific cues and local microenvironments.
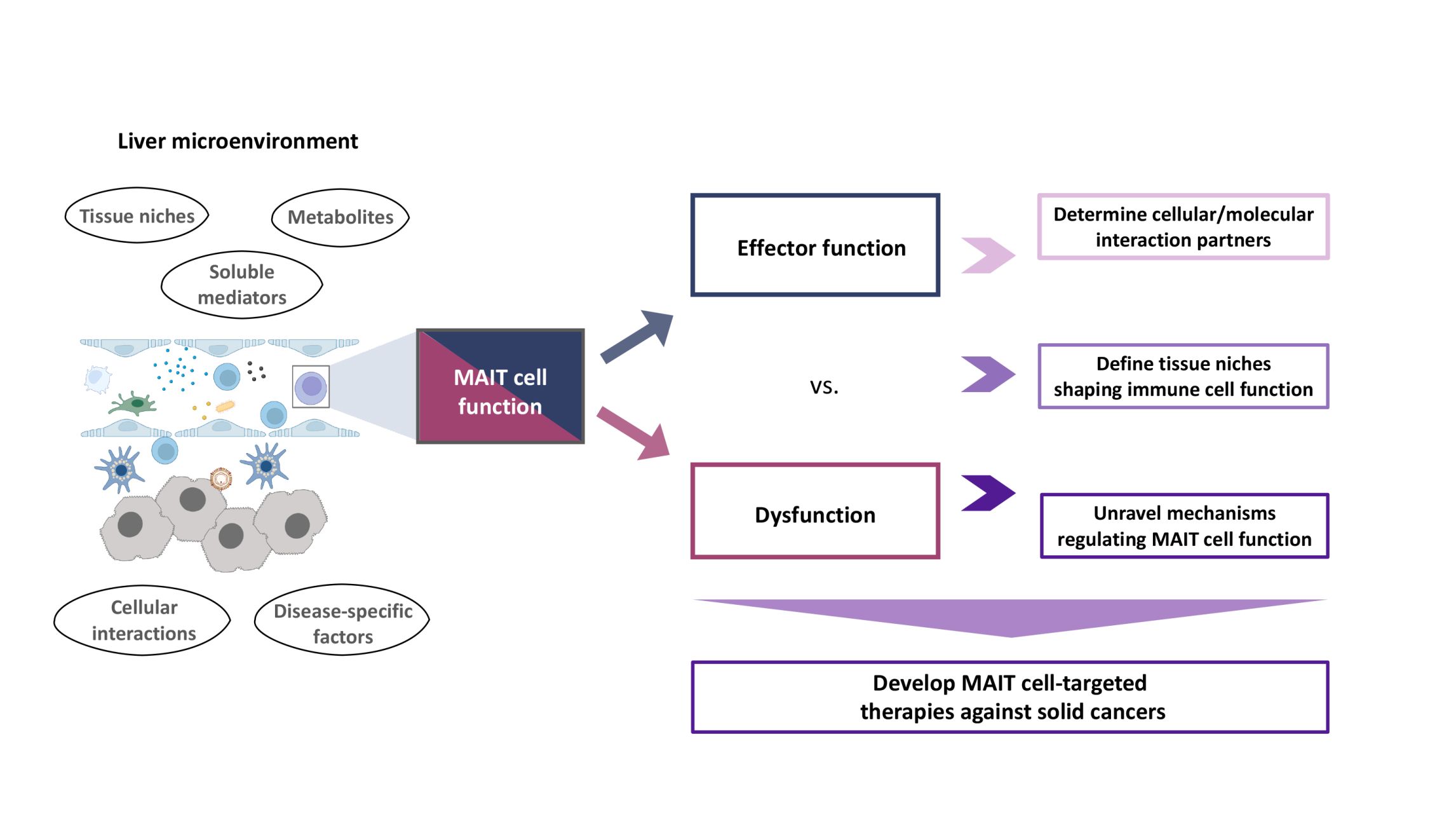
Our aim is to understand mechanisms of immune cell dysfunction in cancer development, progression and resistance to immunotherapy. Primarily focusing on hepatobiliary malignancies, we investigate the contribution of tissue niches, cellular networks, as well as metabolic and molecular pathways to anti-cancer immune responses. Recent work by us and others has highlighted the role of innate-like mucosal-associated invariant T (MAIT) cells for cancer development. Therefore, in collaboration with our partners at the University of Tübingen and beyond, we explore how microenvironmental changes in diseases that predispose to cancer development, such as metabolic diseases and chronic viral infections, affect the role of MAIT cells in cancer development. Further focus areas of the group include spatial analysis of immune cells in human tissues, as well as engineering and manipulation of immune cell function.
Our work stands at the intersection of immunology and clinical application, aiming to advance treatments for hepatobiliary cancers and related hepatological conditions.